Cooking with gas
Methane, ammonia, hydrogen, a dash of iron, a cup of carbonate, then lightning for a little zest. Voila – primordial soup.
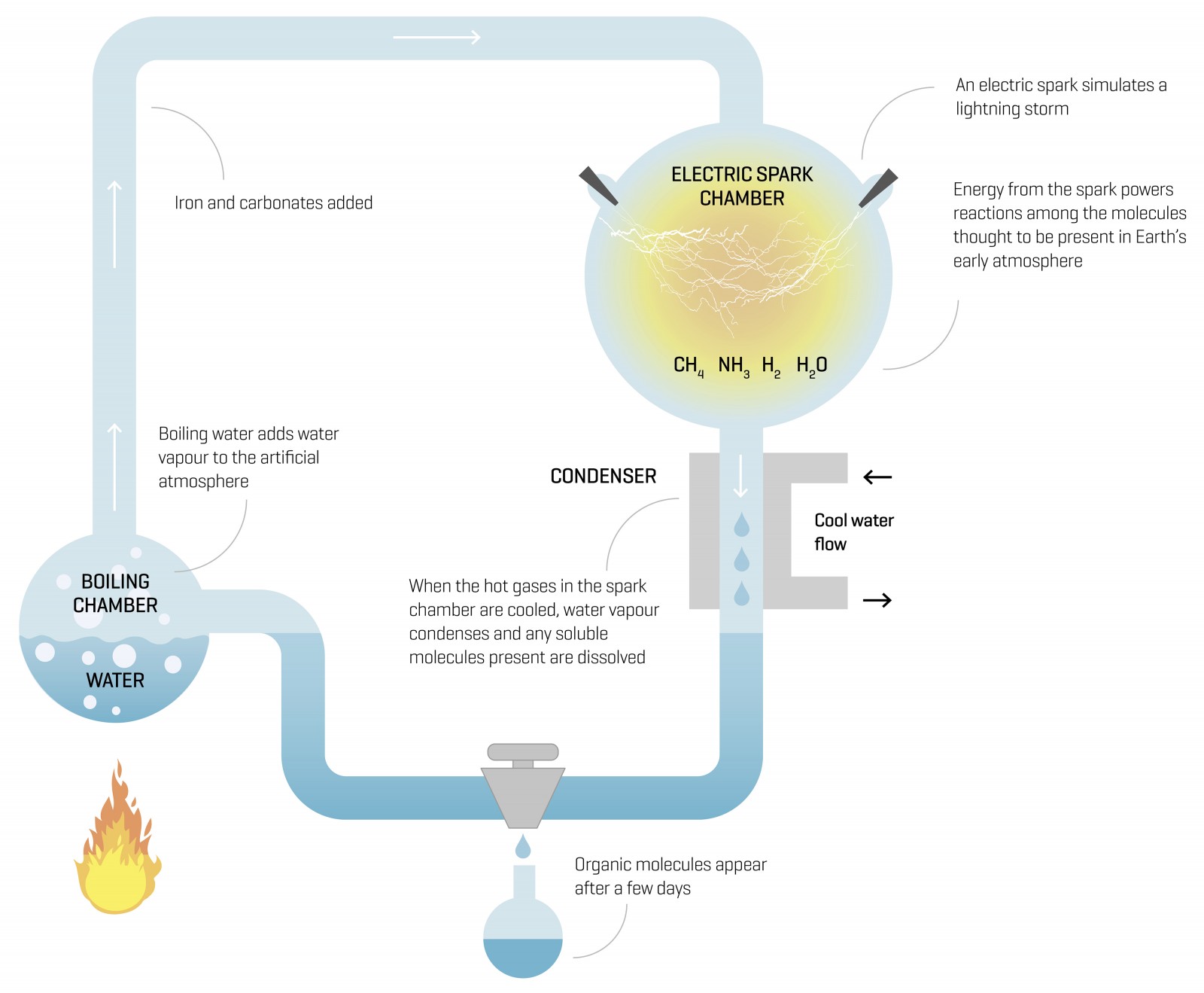
A laboratory. Two men in white coats, transfixed on flasks of broiling chemicals. Electrodes. A flash. Then, one of the great eureka moments. In 1953, University of Chicago graduate student Stanley Miller began mixing a simple cocktail of water, methane, ammonia and hydrogen gas—a distillation, literally, of the Earth’s early, bitter atmosphere.
Under the guidance of his professor, Harold Urey, he fired an electrical charge primordial lightning—through the steaming mix. A day later, the mixture had turned a formative shade of pink. Energised by the electric charge, gas molecules began to transmogrify into compounds such as hydrogen cyanide, aldehydes and ketones. After a fortnight of electro-stimulation, around a quarter of the carbon in the closed loop had assembled into organic compounds. A smaller fraction had formed amino acids mostly glycine. Sugars had appeared.
Amino acids, the hackneyed metaphor goes, are the building blocks of life. In certain combinations, they form proteins that are responsible for many of the structures and functions of the living world.
“If God didn’t do it this way,” breathed Urey as the beakers began to morph green and yellow, “he missed a good bet.”
The pair had apparently created life from nothing, or at least animate from the inanimate. The world got very excited indeed, but it turns out that abiogenesis—the origin of life on Earth—wasn’t quite that simple. How, or whether, life got started here depended critically on the exact composition of our nascent atmosphere, and we know more about it nowadays than could Miller and Urey.
For one thing, modelling and rock analysis have found only limited evidence of hydrogen. Then there’s the irksome revelation that, when entered into contemporary models, carbon dioxide and nitrogen are found to create nitrites. That’s problematic, because nitrites destroy amino acids pretty much the moment they form. But what if the atmosphere were laced also with iron and carbonate minerals? They could well have neutralised the nihilistic nitrites, argue molecular biologists.
Subsequently, University of California chemists Jim Cleaves and Jeffrey Bada (a former graduate student of Miller’s) added iron and carbonates to a re-run of Miller and Urey’s experiment: their beakers ended up brimming with amino acids, demonstrating that they could have formed even in the presence of carbon dioxide and nitrogen.
After Miller’s death in 2007, Bada discovered a number of samples from his original experiments. He decided to analyse them using modern equipment, under a hunch that the techniques available to Miller in the fifties may not have revealed the experiments’ full munificence. Miller had activated these samples with a jet of steam (simulating volcanic activity). Using NASA’s hyper-sensitive mass spectrometers at the Goddard Space Flight Center in Maryland, Bada’s team found 22 amino acids, many more than did Miller. Ten of them had never before been detected in similar experiments.
We know that volcanic eruptions were frequent around four billion years ago, and would have belched carbon dioxide, nitrogen, hydrogen sulphide and sulphur dioxide into the atmosphere. We also know that violent lightning storms are stimulated by volcanic plumes. Miller had reluctantly performed a few experiments in 1958 that added hydrogen sulphide gas (though reluctantly, because it stinks of rotten eggs and made him feel ill). Perhaps that’s why he never wrote up his analysis of the fetid samples, but when Bada re-analysed them, he found amino acids containing sulphur, a highly significant result. One of them, methionine, is a product of the ‘start codon’ in the genetic code. It tells a cell how and when to translate the design for a protein.
It’s this molecule that geoscientists nowadays believe most closely resembles the cocktail of the early atmosphere—mostly carbon dioxide, carbon monoxide and nitrogen, infused with other gases such as hydrogen sulphide, methane and ammonia. (Recent experiments at the Carnegie Institute for Science have shown that organic compounds can also form in hydrothermal vents deep in the ocean floor.) Miller’s most widely publicised experiment may have missed the mark, but his other work has shown that conditions on primitive Earth were at least amenable for prebiotic compounds.
But then, so is much of the solar system: all you need to do is replace lightning with ultraviolet light as the activating charge. In 1969, the Murchison meteorite slammed into Victoria, Australia. When geochemists chipped into it, they found more than 90 different amino acids, 19 of which are already found here, coursing through the veins of terrestrial life. Comets, too, harbour much of the stuff of origin: NASA’s Stardust spacecraft found glycine, the simplest amino acid, when it probed ejecta from comet 81P/Wild-2 in 2004.
Last year, scientists fired projectiles into the sort of icy mixes believed to constitute the core of comets. The idea was to replicate the shock force of a comet striking the surface of primeval Earth. From out of the frigid fog morphed several amino acids—D- and L-alanines, along with non-protein amino acids and their precursors.
When the solar system was still forming, it may have been Jupiter’s gravity that determined the fate of life on Earth—that massive grip held nearby proto-planets apart. The dislocated bodies went on colliding instead, fragmenting into a vast asteroid belt. If the young Jupiter had strayed into that belt, it would have scattered the asteroids. Had it settled into a more distant orbit, asteroids would have been free to pound Earth into a lifeless void. Instead, the fortuitous balance struck meant that just enough asteroids could reach Earth—bearing their organic germs—without rendering oblivion.

Because the atmosphere was thinner then, many more comets and meteors survived intact to pepper proto-Earth during a (still theoretical) galactic rain dubbed The Late Heavy Bombardment, somewhere between 4.1 and 3.8 billion years ago.
Last July, scientists from the University of Sheffield launched sampling balloons into the high stratosphere during a meteor shower and collected ‘biological entities’.
“Life is coming into Earth from space all the time. Immediately you walk outside the house,” study leader Dr Milton Wainwright told BBC Radio, “you’re going to be covered in microrganisms or biological entities coming from space. We believe they’ve been coming in from the year dot.”
Regardless of how they appeared on Earth, amino acids—even complete proteins—are not enough to constitute life. We know still less about what happened next, but the assumption is that those molecules developed into self-replicating organic compounds. Exactly how that happened is still a mystery, but one thing is becoming clear: the means was readily available. The ingredients of life are in widespread circulation, certainly throughout our solar system, probably throughout the Milky Way, and possibly throughout the universe. In 2012, Jes Jorgensen and Jan M. Hollis from Copenhagen University used a radio telescope to detect glycolaldehyde—a sugar molecule—400 light years from Earth.
Glycolaldehyde is an essential component of ribonucleic acid, or RNA, and the Murchison meteorite contained others: uracil and sister molecules including xanthine. Scientists say complex organic solids could be created naturally by stars. A 2011 NASA paper suggested that adenine, guanine and other related organic nucleo bases were synthesised in deep space.
It may be that terrestrial amino acids and proteins didn’t need to find a way to coalesce and replicate: the solution was delivered to them aboard meteors and comets. It’s easy to think of life as a miraculous fluke. But with all that tinder billowing about the universe, something was always going to catch alight. With every new scientific discovery, life is looking more and more like an inevitability.